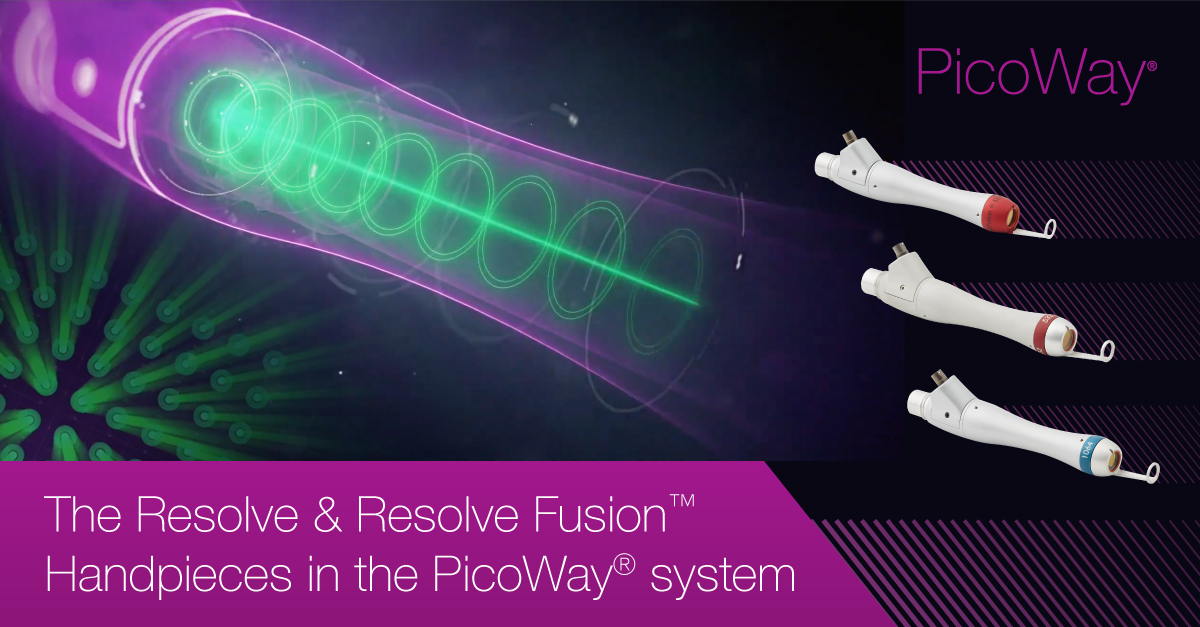
The PicoWay® system offers three fractionated handpieces – the NEW Resolve Fusion™ 532 nm, Resolve 1064 nm, and Resolve 532 nm handpieces which are generated with a unique beams splitter.
The holographic lens allows even distribution of energy with minimal background energy loss to ensure adequate laser energy to be delivered in reaching satisfactory clinical outcomes.

● Shortest pulse duration 532 nm diffractive handpiece†
● 100 distributed spots with minimal background energy loss1
● Build collagen and elastin5 without breaking the epidermis.1,2
● Unique diffractive technology with holographic lens for even treatment
● Laser Induced Optical Breakdown (LIOB) to transform skin
● Use for non-ablative wrinkle reduction and acne scar treatment

● Blended technology with low fluence ring for more coverage^
● Fusion of high fluence central beam and low fluence diffuse enhances the coverage the coverage up to 50%2
● 5x more coverage per pass† for potentially saving treatment time3 & minimizing risk of Post-inflammatory Hyperpigmentation 4
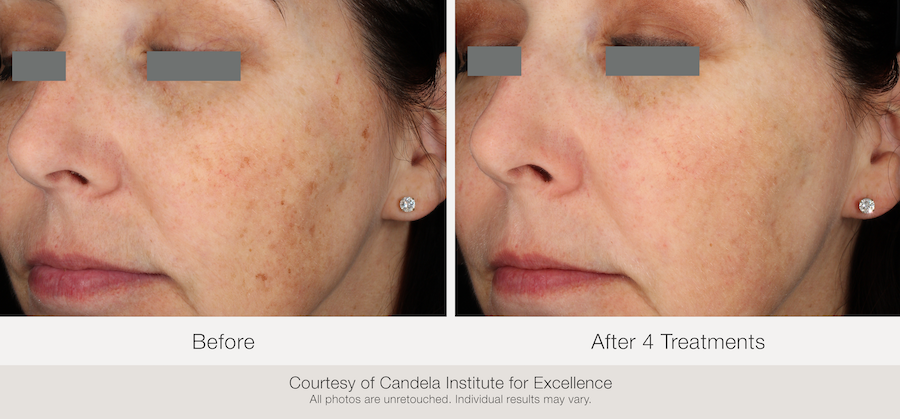
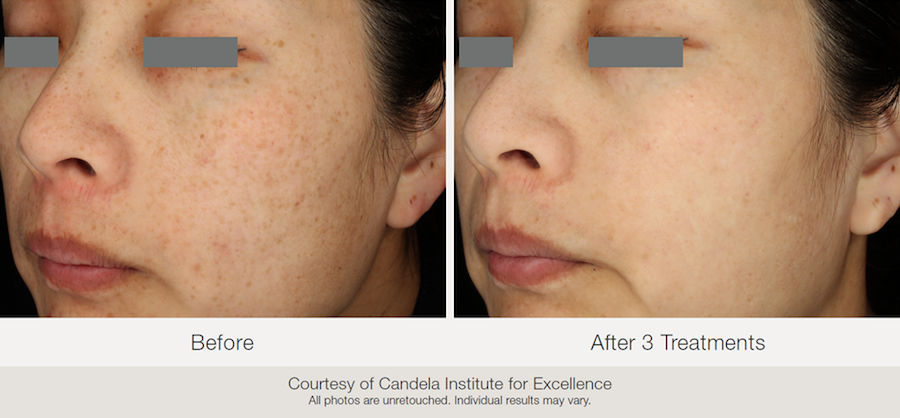
All photos are courtesy of Candela Institute for Excellence.
All photos are unretouched. Individual results may vary.
†Based on published competitor’s specifications with FDA clearance
^Claims compared to the PicoWay® Resolve 532 nm handpiece
References:
1. PicoWay 510(k) clearance (K191685), September 2019. 2. Candela, Data on File (compared to 10% of the PicoWay® Resolve 532 nm coverage) 3. K. Khatri MD, K Patel MD. Candela Institute for Excellence, Marlborough MA. Candela. Data on File. 2019. 28 Agbai O, et al. JAMA Dermatol. 2017;153(2):199-206. 4. Schomacker K, Bhawalkar JD. PicoWay Clinical Bulletin. 2016. Data on file
Download your complimentary copy of PicoWay® Resolve Handpieces Info Package today to learn more about them!
A copy will be sent to your inbox momentarily.
Disclaimer: The views and opinions expressed in this panel discussion are those of the authors and do not necessarily represent the official position of Candela Corporation or its affiliates. All contents of this material are for informational purposes only and provided by Candela Asia-Pacific without warranties of any kind. Healthcare professionals are solely responsible for making their own independent evaluation as to the suitability of any product for any particular purpose and in accordance with country-specific regulations. The availability of products and the indications mentioned in this material is subject to the regulatory requirements and product registration status in each country. Refer to the User Manual for country-specific indications. Products and technical specifications may change without notice. Please contact Candela Asia-Pacific for more details.
©2022 Candela Corporation. This material contains registered and unregistered trademarks, trade-names, service marks and brand names of Candela Corporation and its affiliates. All other trademarks are the property of their respective owners. All rights reserved.
NOT ALL PRODUCTS ARE LICENSED IN YOUR COUNTRY, PLEASE CHECK WITH YOUR LOCAL REPRESENTATIVE. ALL PERSONAL DATA SUBMITTED TO CANDELA VIA THIS WEBSITE WILL BE HELD IN ACCORDANCE WITH OUR PRIVACY NOTICE, WHICH CAN BE VIEWED HERE. © 2020 CANDELA CORPORATION. ALL RIGHT RESERVED.